Roni P. Dodiuk-Gad; Wen-Hung Chung; Laurence Valeyrie-Allanore; Neil H. Shear
Am J Clin Dermatol. 2015;16(6):475-493.
Abstract and Introduction
Abstract
Stevens–Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) are life-threatening mucocutaneous reactions, predominantly drug induced. The mortality rates for SJS and TEN are as high as 30 %, and short- and long-term morbidities are very common. SJS/TEN is one of the few dermatological diseases that constitute a true medical emergency. Early recognition and prompt and appropriate management can be lifesaving. In recent years, our understanding of the pathogenesis, clinical presentation, and management of SJS/TEN has improved. Nevertheless, in 2015, there are still no internationally accepted management guidelines. This review summarizes up-to-date insights on SJS/TEN and describes a protocol for assessment and treatment. We hope these suggested guidelines serve as a practical clinical tool in the management of SJS/TEN. The classic manifestation of SJS/TEN consists of initial ''flu-like'' symptoms (malaise, fever, anorexia) in the prodromal phase, followed by cutaneous and mucous membrane (ocular, oral, and genital) inflammation and pain, and other systemic involvement. Symptoms usually begin 4–28 days after the onset of drug intake. Treatment is multidisciplinary and includes identification and withdrawal of the culprit drug, transfer to a specialist unit, supportive care, medical treatment, communication, and provision of appropriate information and emotional support.
1 Introduction
Stevens–Johnson syndrome (SJS) was first described by A. M. Stevens and F. C. Johnson in 1922.[1]The term ''toxic epidermal necrolysis'' (TEN) was coined in 1956 by A. Lyell.[2] Neither of those publications truly described what today is considered to be SJS or TEN. There are clinical and pathological variants of both SJS and TEN, but the terms are also used to define a condition that is a spectrum of disease from SJS to TEN. SJS and TEN represent different degrees of the same type of severe cutaneous adverse reaction. The mortality rates for SJS and TEN are high; that of TEN may approach 30 %.[3] In this manuscript, we refer to this specific disease spectrum as a single entity—namely, SJS/TEN.
In recent years, our understanding of the pathogenesis, clinical presentation, and management of SJS/TEN has been significantly enlightened. This review aims to summarize the most up-to-date insights.Continue Reading
Disclosures
Am J Clin Dermatol. 2015;16(6):475-493.
 Print
Print
- Abstract and Introduction
- 2 Pathogenesis
- 3 Clinical Presentation
- 4 Assessment and Treatment of SJS/TEN
- 5 Conclusion
- References
- Sidebar
2 Pathogenesis
SJS/TEN mostly results from a cumulative effect of aligned risks related to the structure of a drug and to the patient's genetic predisposition (human leukocyte antigen [HLA] alleles, drug metabolism characteristics, and T cell clonotypes) (Fig. 1).
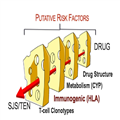
(Enlarge Image)
Figure 1.
''Swiss cheese'' risk model of Stevens–Johnson syndrome and toxic epidermal necrolysis (SJS/TEN). CYP cytochrome P450, HLA human leukocyte antigen
2.1 Genetic Associations of SJS/TEN
Over the past few decades, important progress has been made in understanding the pathogenic mechanisms of SJS/TEN—specifically, the important role of HLA alleles. The pathogenesis of inducing cytotoxic responses in SJS/TEN is generated by recognition of culprit drugs by specific HLA molecules. In 1987, the genetic susceptibility of HLA alleles was first proposed by Roujeau et al.,[4] who identified weak associations of HLA-B*12 and oxicam- and sulfonamide-related TEN in Europeans. More notable evidence of genetic susceptibility to SJS/TEN was reported in 2004 by Chung et al.,[5] who found thatHLA-B*15:02 is strongly associated with carbamazepine (CBZ)-induced SJS/TEN. The early associations were made in an era of serological typing of HLA. Currently, in an era of sequence-based typing and deep sequencing, which allows resolution to four digits, identification of more specific and stronger associations are possible. Hence, many other HLA associations in SJS/TEN have been discovered,[4, 6–21] as are detailed in Table 1.
HLA associations with specific drug-induced SJS/TEN can be restricted to certain ethnicities and phenotypes. The strength of HLA associations with specific drug-induced SJS/TEN has been found to be related to the prevalence of the susceptibility allele in the ethnic population. The association of HLA-B*15:02 and carbamazepine-induced SJS/TEN has been reported in Han Chinese, Thai, Indian,[22] and Malaysian populations[23] but not in Europeans, who carry the HLA-B*15:02 allele in low frequency (<1 %).[24] The association is also present in Han Chinese ancestry of Europeans. This ethnic difference can be tracked by historical evolution. The US Food & Drug Administration and Asian health administrations have recommended HLA-B*15:02 screening for new carbamazepine users of Asian ancestry since 2007.[25] In contrast, the HLA-B*58:01 allele with allopurinol-induced SJS/TEN is common to all populations, being found in Han Chinese, Thai, Japanese, Korean, and European populations.[26]
There are also phenotype-specific characteristics involved in carbamazepine hypersensitivity. HLA-B*15:02 and other B75 serotype HLA alleles are strongly associated with carbamazepine-induced SJS/TEN but are not associated with carbamazepine-induced drug reaction with eosinophilia and systemic symptoms (DRESS); however, HLAA* 31:01 is strongly associated with carbamazepine-induced DRESS but less so with carbamazepine-induced SJS/TEN.[27]
Association with a specific HLA risk allele appears to be necessary but not sufficient for development of SJS/TEN. Additional factors such as individual differences in drug metabolism or clearance may also play an important role in SJS/TEN development, recovery, or prognosis. Drug clearance is known to be important for preventing exceptional damage, which can occur as a result of retention of the drug in the body. Shear and Spielberg first recognized potential pharmacogenetic associations and risks of altered drug metabolism for SJS/TEN and DRESS with anticonvulsants and sulfonamides.[28,29]
A recent genome-wide association study by Chung et al.[30] reported that genetic variants of cytochrome P450 2C (CYP2C) are strongly associated with phenytoin-related SJS/TEN. They identified 16 significant single-nucleotide polymorphisms (SNP) in CYP2C genes at 10q23.33. Further studies showed that CYP2C9*3 variants, which reduce CYP2C9 enzymatic activity, were significantly related to phenytoin-induced SJS/TEN. CYP2C9*3 has been known to be related to the drug's metabolism and can attenuate the clearance of phenytoin.[31,32] Patients with phenytoininduced SJS/TEN who carriedCYP2C9*3 showed delayed clearance of plasma phenytoin, resulting in increasing phenytoin toxicity in the body. Another study showing that genetic variability in a metabolizing enzyme can contribute to SJS/TEN examined nevirapine-induced SJS/TEN. CYP2B6 G516T and T983C SNPs were found to be associated with SJS/TEN susceptibility.[33] The issue of drug metabolism or clearance is another key factor in consideration of the risk of developing SJS/TEN.
2.2 Immunological Mechanisms of SJS/TEN
SJS/TEN is mostly recognized as an immune disorder elicited by drugs. SJS/TEN is a delayed-type drug hypersensitivity reaction, with a typical latency of 4–28 days and with cases rarely occurring as long as 8 weeks following initiation of the implicated drug.[34]
The drugs are of low molecular weight and often serve as foreign antigens that are recognized by T cell receptors (TCRs) to activate adaptive immune responses. Several proposed concepts have been found to explain how small-molecule compounds are recognized by TCRs. In some cases, drugs interact directly with the TCRs involved in presenting to HLA molecules of antigen-presenting cells (APCs). This model is known as the p-i [pharmacological interaction of drugs with immune receptors] concept.[35] An example is that carbamazepine cannot bind covalently to peptides or proteins but is able to bind with low affinity to TCRs and provoke T cell activation.[36,37] Drugs can also interact with TCRs by a drug–peptide complex presented to HLA molecules of APCs in what is known as the hapten concept. The b-lactams that form covalent binding to lysine residues are an example.[38] Upon noncovalent binding of specific drugs presented to HLA molecules and TCRs, the HLA–drug–TCRs may initiate a series of immune reactions, which result in activation of CD8+ cytotoxic T cell–mediated and natural killer (NK) cell–mediated cytotoxicity. Recently, the shared and restricted TCR usage subtype in carbamazepine-induced SJS/TEN was identified,[39] and it was demonstrated that the endogenous peptide-loaded HLA-B*15:02 molecule presented carbamazepine to cytotoxic T cells without the involvement of intracellular drug metabolism or antigen processing (Fig. 2).[37]
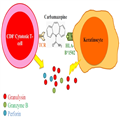
(Enlarge Image)
Figure 2.
Direct interaction between the HLA-B*1502 molecule and carbamazepine activates the T cell with a restricted T cell receptor (TCR). HLA human leukocyte antigen
Cytokines are involved in the pathogenesis of SJS/TEN. Several studies have shown that tumor necrosis factor (TNF)-α is strongly expressed in SJS/TEN lesions and correlates proportionally with disease development.[40–42] TNF-α can induce cell apoptosis, activation, and differentiation, and an inflammatory response.[43, 44] In addition, interferon (IFN)-γ is a common cytokine involved in delayed-type drug hypersensitivity, including SJS/TEN. IFN-γ is often expressed in the superficial dermis and epidermis of SJS/TEN lesions.[41,42] IFN-γ is known to induce antigen presentation and thus stimulate cell-mediated immunity by upregulation of HLA molecules.[45–47] TNF-α, IFN-γ, several cytokines, and chemokine receptors that are responsible for proliferation, trafficking, and activation of T cells have been found in the skin lesions, blister fluids, blister cells, peripheral blood mononuclear cells, or plasma of SJS/TEN patients. These cytokines/chemokines include interleukin (IL)-2, IL-5, IL-6, IL-10, IL-12, IL-13, IL-15, IL-18, chemokine (C–C motif) receptor (CCR) 3, chemokine (C-X-C motif) receptor (CXCR) 3, CXCR4, and CCR10.[40–42, 48–50]
The major theory to explain the severe epidermal detachment of SJS/TEN is CD8+ cytotoxic T cell–mediated and NK cell–mediated cytotoxicity. It has been well established that epidermal detachment in SJS/TEN is due to keratinocyte apoptosis. Recently, studies have shown that keratinocyte apoptosis in lesional skin and blister fluid in SJS/TEN patients is associated with greatly increased numbers of CD8+ cytotoxic T cells and NK cells.[51, 52] While CD8+ cytotoxic T cells and NK cells are activated, they subsequently carry out the cellular-mediated immune reactions directed at keratinocytes in an HLA class I–restricted manner. Upon activation of these responses, various cytotoxic signaling molecules, including granulysin, perforin/granzyme B, and Fas/Fas ligand, are relayed to the skin lesions to induce keratinocyte apoptosis.[52–54] More importantly, Chung et al. found that granulysin—a cytotoxic protein produced by cytotoxic T cells or NK cells—acts as a key mediator responsible for disseminated keratinocyte death.[52, 55] Furthermore, granulysin is not only a cytotoxic protein but also a chemoattractant and proinflammatory activator, which can promote monocyte expression of chemokine (C–C motif) ligand (CCL) 20[56] and is capable of promoting antigen-presenting (dendritic) cell and leukocyte recruitment (specifically, a granulysin 15 kD subunit, which is largely produced by CD8+ T cells and NK T cells).[57] Granulysin-positive cells in fixed drug eruptions have been found to be similar to those observed in SJS/TEN.[58]
2.3 Environmental Factors
SJS/TEN can be secondary to infection with Mycoplasma pneumoniae or herpes simplex virus, but the full pathogenesis remains unclear.[59–61] Human enterovirus has not previously been recognized to be associated with SJS.[59,60] By comparison, erythema multiforme major (EMM) is mainly caused by viruses, usually involving the palms and soles, and the patient experiences rapid healing without sequelae.
A recent study found that a new variant of coxsackie virus (CV) A6, which belongs to the human Enterovirus genus and causes severe mucocutaneous blistering reactions, mainly mediated by cytotoxic T cells and NK cells expressing granulysin, mimics the histopathological features of SJS or EMM in children.[62] In fact, there are still about 20 % of SJS/TEN cases without an identified causality.[63,64]Potential risk factors for this unusual presentation of virus infection as a cause of SJS/TEN have not been elucidated.Continue Reading
3 Clinical Presentation
3.1 Acute Stage
Initially, SJS/TEN begins within 4 weeks (usually 4–28 days) after the onset of drug intake.[65] The disease can also occur a few days after the drug has been withdrawn in the case of long-half-life drugs. In very rare cases of rechallenge with the same drug, the disease appears more rapidly, within hours.[66]
Initial symptoms are usually non-specific and can precede cutaneous manifestations by a few days (1–3 days) in one third of cases. Painful mucous membranes, stinging eyes, headache, rhinitis, malaise, cough, sore throat, and myalgias are frequently noticed. In the absence of cutaneous manifestations, these symptoms may contribute to an erroneous initial diagnosis and delayed specific management. In other cases, the disease may begin with mucous membrane involvement and/or non-specific exanthema. However, the addition of new clinical signs, severe pain, and rapid progression should alert the physician and may lead to consideration of a severe disease.
3.1.1 Cutaneous Lesions. Early sites of cutaneous involvement are the presternal region of the trunk, the face, and proximal parts of the limbs. Palms and soles may also be initially involved. The rash may spread to distal parts of the limbs and the rest of the body within a few days. Individual cutaneous lesions appear as erythematous, dusky dark red purpuric macules, which are irregularly shaped (Fig. 3).[67, 68] These atypical targets, with two concentric rings, have a necrotic center and may tend to coalesce.[68] At this stage, involvement of at least two mucous membrane sites is observed in up to 90 % of cases, and this feature may help in diagnosis.
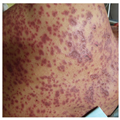
(Enlarge Image)
Figure 3.
Erythematous dusky red macules on the trunk
In the absence of spontaneous detachment, a positive Nikolsky sign should be sought by exertion of lateral mechanical pressure with a finger on an erythematous zone.[67, 68] This sign is considered positive if dermal–epidermal cleavage is induced and necrotic epidermis detachment on pressure points reveals large areas of exposed, red, sometimes oozing dermis. It can also be observed in autoimmune blistering diseases such as pemphigus. According to the percentage of epidermis that is detached or detachable, patients are classified into one of three groups: SJS involves <10 % of body surface area (BSA); SJS/TEN overlap involves between 10 % and 30 % of BSA; TEN involves >30 % of BSA.[68]
Despite the obvious differences from erythema multiforme, occasional typical target lesions may appear.[67, 69] These lesions rapidly extend within 4 to 5 days. Skin involvement can be limited to the predilection sites at the SJS end of the spectrum (face, trunk) but are widespread in TEN. TEN displays areas of diffuse erythema, with individual macular lesions at the periphery. The epidermis detaches from the underlying dermis, leading to large, flaccid blisters (Fig. 4). The roofs of the blisters turn necrotic and display denudation of the epidermis in large sheets measuring >5 cm (Fig. 5). Tense bullous lesions are more frequently observed at the initial stage and on palmoplantar soles.

(Enlarge Image)
Figure 4.
Bullous lesions due to epidermal necrosis
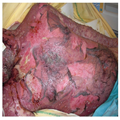
(Enlarge Image)
Figure 5.
Blisters and epidermal detachment have led to large confluent Erosions
Thermal burn rules are currently used in SJS/TEN. Evaluation of the BSA involved may be difficult and is often overestimated, more particularly in the case of spotty lesions.
3.1.2 Mucous Membranes. Involvement of mucous membranes is noticed in more than 80 % of cases with at least two sites involved and may be inaugural in one third of cases.[68] Painful inflammation and erosions of mucosal surfaces occurs in 87 to 100 % of cases of TEN.[70, 71] Lesions usually begin with painful, burning sensations of the lips, conjunctivae, and genitalia, followed by edematous, erythematous, and flaccid bullous lesions. Blisters rupture and tend to extend. Bullous lesions become very painful and hemorrhagic erosions, coated by greyish-white pseudo-membranes of the oral cavity (Fig. 6), leading to impaired alimentation and hypersalivation. Genital erosions most often include painful erosions of the glans penis, vulva, and vagina, and may lead to ''burning'' on micturition, urinary retention, and synechiae.[72] Anal lesions are less frequent. Ocular involvement is mainly represented by conjunctival involvement in 80 % of cases,[73, 74] including photophobia, pain, lacrimation, chemosis, and redness.[73, 74] However, more severe involvement leads to corneal ulceration, anterior uveitis, and purulent conjunctivitis. Ultimately, this can lead to blindness.

(Enlarge Image)
Figure 6.
Extensive erosions of the lips and oral mucosa
3.1.3 Systemic Manifestations. SJS/TEN corresponds to an acute skin failure associated with severe weakness, pain, and prolonged high fever.[75] Internal epithelial organ involvement is rare and mainly concerns the respiratory and gastrointestinal tracts. Early pulmonary dysfunction is observed in 25 % of cases, and the respiratory rate and blood oxygenation must be systematically evaluated.[76] Pulmonary involvement includes breathing difficulties, cough, bronchial obstruction, and respiratory distress. A recent retrospective study reported that 39 % of patients with SJS/TEN had specific endobronchial lesions and 25 % required mechanical ventilation.[77] Initial bronchial hypersecretion, laryngeal involvement, and dyspnea should alert the physician.
Less commonly, gastrointestinal involvement has been reported, including diarrhea, abdominal distention, and excretion of colonic intestinal epithelium, which can lead to bowel perforation.[78, 79]
Renal involvement is currently observed in the acute phase, mainly represented by acute renal failure, proximal acute tubular necrosis, hematuria, and microalbuminemia.[80] Anemia, mild elevation in hepatic enzymes and amylase (mostly of salivary origin) are frequent, without any impact on prognosis. Neutropenia is rare and is considered a severity marker but is too rare to have an impact on the Severity of Illness Score for TEN (SCORTEN) (Table 2).
3.1.4 Prognosis. Progression lasts for about 4–5 days following admission. Next, patients enter a plateau phase, which corresponds to progressive re-epithelialization. Complete healing can take a few days to several weeks. This phase is associated with life-threatening complications such as sepsis due to epithelial loss.[81, 82]Staphylococcus aureus and Pseudomonas are the most frequent pathogens involved, both usually present in the skin and blood. Multisystem organ failure and pneumonitis are important causes of morbidity, with prevalence rates of 24 and 23.1 %, respectively.[82]
In Europe, according to the RegiSCAR (European Registry of Severe Cutaneous Adverse Reactions) results, the mortality rate was 23 % at 6 weeks and 34 % at 1 year.[83] The severity of the reaction was a risk factor for mortality only in the 3 months after onset, whereas serious comorbidities and age influenced mortality beyond 90 days and up to 1 year after the onset of the reaction.[83]
In parallel, it has already been demonstrated that prompt withdrawal of the offending agent may reduce the risk of death by 30 %.[84]
A SJS/TEN-specific severity-of-illness score is proposed (SCORTEN), in which seven variables remain independent prognosis factors of death: age >40 years, heart rate >120 beats/min, cancer or hemopathy, BSA involvement >10 %, serum urea level >10 mmol/L, serum bicarbonate level <20 mmol/L, and serum glucose level >14 mmol/L (Table 2).[85] The score's ability to predict outcome and usefulness has been confirmed by several teams,[86, 87] and a simplified score has recently been proposed for cases with missing laboratory data.[88] The prognostic value of SCORTEN is more accurate at day 3 of hospitalization.[89]
3.2 Chronic Stage
During the past few decades, SJS/TEN has no longer been considered as only an acute disease. Specific follow-up of sequelae should be systematically planned and studied for better understanding of the pathophysiology, detection, and prevention of the reaction, and to reduce the health burden.[90]Dermatological sequelae frequently include hyper-and hypopigmentation (72 %), hypertrophic scars, and nail dystrophies (pigmentation in the nail bed, ridging, and permanent anonychia).
In 65–89 % of cases, patients develop late ocular complications, including dry eye syndrome, trichiasis, photophobia, symblepharon, corneal inflammation and neovascularization, and, in the most severe cases, reduced visual acuity and blindness.[73, 91]
Buccal and dental complications in a study of 16 patients with TEN reported gingival synechiae, gingival recession, dental alteration, xerostomia, and increased saliva acidity.[92]
Long-term follow-up of SJS/TEN patients can identify chronic lung disease, mostly bronchiolitis obliterans. Pulmonary function tests performed during the usual followup display abnormalities presenting mainly as asymptomatic diffusion impairment, with a risk proportional to BSA involvement.[93]
Male genitalia synechiae may require circumcision. Strictures of vaginal mucosa and/or birth-canal stenosis[72] may be responsible for dyspareunia or vaginal dryness, and can complicate spontaneous vaginal delivery and sexual intercourse. Surgical, topical, physical, and laser treatments are often required.
The results of our recent study assessing the long-term emotional and physical sequelae of SJS/TEN[94]demonstrated major emotional complications (symptoms of anxiety, depression, and post-traumatic stress), impaired healthrelated quality of life, and long-term physical complications (most commonly cutaneous and ophthalmic).Continue Reading
4 Assessment and Treatment of SJS/TEN
SJS/TEN is one of the few dermatological diseases that constitute a true medical emergency. Early recognition and prompt appropriate management can be lifesaving. Nevertheless, there are no internationally accepted management guidelines. The following represents the authors' protocol for assessment (Table 2) and treatment (Table 3) of patients with SJS/TEN, based on the most up-to-date literature and abundant experience in treating patients with SJS/TEN. We hope these suggested guidelines may serve as a practical clinical tool for physicians in the management of patients with SJS/TEN. While this is international by definition, it is not necessarily a consensus-based protocol. For the readers' convenience, we have designed two tables: the assessment protocol (Table 2) and the treatment protocol (Table 3), which summarize the key elements.
4.1 Assessment
Assessment is conducted by the simple method of ''5Ds by Dr. Shear''. Specifically, these are Diagnosis,Drug exposure, Differential diagnosis, Determining the probabilities of causality, and Determining the severity of SJS/TEN.
4.1.1 Diagnosis. The physician must determine whether the patient's clinical symptoms are signs of SJS/TEN or of another skin disease. The diagnosis is based on three key clinical elements: cutaneous and mucous membrane manifestations, systemic involvement, and histological findings. A thorough history and a comprehensive physical examination are essential for accurate diagnosis of SJS/TEN. Basically, the classic manifestation of SJS/TEN consists of initial signs of ''flu-like'' symptoms (malaise, fever, anorexia) in the prodromal phase, followed by cutaneous and mucous membrane inflammation and pain (ocular, oral, and genital) and other systemic involvement (see elaboration in Sect. 3).
For cutaneous assessment, the classification of SJS/TEN defined by Bastuji-Garin et al.[69] includes the proper definition of the characteristic skin lesions. For BSA involvement, the Wallace rule of nines can be used; a value of 9 % of BSA is given for involvement of the head, each arm, the chest, and the abdomen; 18 % is given for each leg and the back; and 1 % is given for the groin. Also, the patient's palm can serve as a reference point roughly equivalent to 1 % of BSA for total assessment. For children and infants, the Lund and Browder chart is used to assess the burned BSA. The extent of epidermal detachment should also be assessed. It should be emphasized that only necrotic skin that is already detached (e.g. blisters, erosions) or detachable skin (a positive Nikolsky sign, whereby slight rubbing of the skin results in exfoliation of the outermost layer) should be included in the evaluation of the extent of epidermal detachment.[61]
Histological assessment is a key element in the diagnosis of SJS/TEN and to rule out diseases that may mimic SJS/TEN. Characteristic histological features include extensive keratinocyte destruction with separation of the epidermis from the dermis at the dermoepidermal junction.[95] A skin biopsy for direct immunofluorescence (DIF) from perilesional skin should also be conducted for the differential diagnosis. Blood work and consultation with specialists (ophthalmology, otolaryngology, and gynecology) for comprehensive assessment of mucous membrane involvement are to be conducted in the primary assessment of the patient, according to the clinical signs. During hospitalization, ocular assessment should be conducted on a daily basis, and consultation with other specialists is based on the patient's clinical signs and symptoms.
A rapid immunochromatographic test for serum granulysin was found to be useful for the prediction of SJS/TEN[96] and, in the future, it may serve as a clinical tool for early diagnosis. However, it is still not known if granulysin may also be useful as a prognostic marker for SJS/TEN.
4.1.2 Drug Exposure (Timing). All medications, regardless of the route of administration, must be considered, especially new drugs taken in the 8 weeks prior to the skin reaction. Drugs taken intermittently, such as vitamins, sedatives, pain relievers, laxatives, and natural products, must also be considered. It is also important to avoid a common mistake, which is to falsely implicate drugs that were introduced for early symptoms of SJS/TEN, such as antipyretics or antibiotics, in the causality of SJS/TEN. Assessment of the lag period—the time between initiation of the drug and the onset of the cutaneous reaction—is crucial in view of the different lag times for different cutaneous drug reactions. A recommended method for drug exposure analysis is to chart a timeline of the patient's illness in order to visualize the chronology and facilitate comprehension of the event. The timeline includes the relevant information (the starting day, dosage, and discontinuing day) for each drug and the signs and symptoms throughout the period in question.[97, 98] In SJS/TEN, the lag period is 4–28 days. The median latency was found to be longer ( >30 weeks) for drugs with no associated risk.[65]
4.1.3 Differential Diagnosis. Establishing a differential diagnosis that takes into account all possible diagnoses is essential. Ranking of the approximate likelihood of each condition is encouraged. The major differential diagnoses for SJS/TEN are detailed in Table 2. The following clues may assist in differentiating them.
Staphylococcal Scalded Skin Syndrome (SSSS): In addition to the different clinical findings (typically the lack of mucous membrane involvement in SSSS), performing a frozen section to evaluate the level of epidermal separation, which is intra-epidermal in SSSS but sub-epidermal in TEN, is a quick tool for differentiating between these diseases.[99]
Generalized Bullous Fixed Drug Eruption (GBFDE): The following typical clinical features of GBFDE may aid in differentiating it from SJS/TEN: (1) blistering usually affects only a small percentage of BSA, and between the large blisters there are sizable areas of intact skin; (2) erosive mucosal involvement is rare and, when it does occur, is rather mild; (3) patients usually do not feel sick or have fever, and generally they are in much better overall health than those with SJS/TEN; (4) most patients report a history of a similar, often local reaction.[99]
Acute Graft-Versus-Host Reaction (aGVHR): aGVHR is a multi-organ disease process (major organs include the skin, liver, and gastrointestinal tract) resulting from interactions of donor-derived T cells against antigens expressed on the cells of the recipient host. Severe cases of aGVHR present with symptoms similar to those of SJS/TEN (clinical and histological): diffuse erythroderma, desquamation, bullae, a positive Nikolsky sign, and ulcerations of mucous membranes. Histologically, grade IV aGVHR involves full-thickness epidermal necrosis and has histological findings identical to those in SJS/TEN. The clinical setting assists in differentiation of aGVHR from SJS/TEN. aGVHR occurs in patients with bone marrow and allogeneic hematopoietic stem cell transplants, usually 2 weeks post-transplantation.[100]
TEN-Like Lupus Erythematosus or Lupus-Associated TEN (TEN-Like LE): This was first described in 2003.[101] It is a subacute or acute cutaneous rash, followed by bullous lesions and epidermal cleavage. It has been reported to occur in patients with established systemic lupus erythematosus, and it is considered to result from aggressive inflammatory epidermal basal layer damage due to increased extension of the interface dermatitis characteristic of lupus erythematosus. In distinction to SJS/TEN, there is a lack of an apparent trigger and an absence of systemic and mucous membrane involvement.[102]
4.1.4 Determining the Probabilities of Causality by the Suspected Drugs. Drug exposure is the most common cause of SJS/TEN,[61] with more than 200 culprit drugs identified.[3] The groups of medications associated with a high risk of inducing SJS/TEN vary according to the population (Table 4). Nonmedication triggers, implicated mainly in SJS, include infections, contrast media, and vaccinations.[59, 103, 104] The most significant challenge in assessing SJS/TEN is determining the probabilities of causality between the suspected drugs and the untoward clinical event.
The following methods are helpful:
Patient History: The lag period (the time between initiation of the drug and the onset of the cutaneous reaction) and personal and familial previous cutaneous reactions to drugs are crucial factors in assessing likelihoods.[97]
Analysis of the Literature: Search for information regarding the frequency with which the type of reaction is related to a particular drug (PubMed at http://www.ncbi.nlm.nih.gov/pubmed/; Litt's Drug Eruptions and Reactions Manual and database at http://www.drugeruptiondata.com/).
Algorithm of Drug Causality for Epidermal Necrolysis (ALDEN): This is an algorithm for the assessment of drug causality in SJS/TEN, developed by the RegiSCAR Study Group, and consists of six parameters according to which the drug causality is classified as very unlikely, unlikely, possible, probable, or very probable.[105]
Adjunctive Laboratory Assessments: In this decade, there has been a successful translation of the laboratorybased discovery of the HLA alleles associated with severe cutaneous adverse reactions to drugs to a clinical guidelinebased test.[25] These tests are best utilized as genetic screening tools prior to drug prescription. However, they may also serve as a simple, fast, safe, and reliable method of establishing clear causality between a drug and a disease in cases of SJS/TEN (but should never be used in isolation as a single test in causality assessment) and are an important tool in counseling both the patient and his/her family members about drug avoidance.[106] The HLAs are specific to a drug and an ethnic background[107] (see elaboration in Sect. 2 and Table 1). In vitro assessments such as the lymphocyte transformation test (LTT)[108] and granulysin expression[109] have been reported. However, caution should always be exercised regarding false positive and false negative results. In addition, at this point in time, no laboratory tests should be used as justification for rechallenge when SJS/TEN associated with a drug is clinically possible.
4.1.5 Determining the Severity. The severity of SJS/TEN depends mostly on the haemodynamic status and the extent of systemic involvement, determined by epidermal barrier breakdown symptoms such as hypothermia, dehydration, secondary infection, and organ involvement induced by necrosis of epithelial lining, such as respiratory distress syndrome, colitis, hepatitis, and nephritis (see elaboration in Sect. 3).[110] This assessment is conducted by taking a thorough history, a physical examination, blood work, and proper imaging (a chest X-ray is advised for all patients; further imaging is based upon clinical findings).
The following clinical and histological findings were found to be validated values for determination of severity.
SCORTEN: This scoring system was developed to stratify the severity of illness and predict mortality in patients with TEN. To optimize the predictive value of this tool, SCORTEN is to be performed on days 1 and 3 post-admission (see elaboration in Sect. 3 and Table 2).[85]
Histological Findings: In a retrospective analysis of the clinical records and histological material from 37 patients with TEN, the extent of dermal mononuclear inflammation was found to predict the clinical outcome approximately as well as SCORTEN. Increased inflammation correlated with a worse prognosis; a mean dermal mononuclear cell count >215 cells per high-power field in patients with 30 % or more total BSA sloughing predicted a worse prognosis (65 % mortality), compared with 24 % mortality in those with<215 cells per high-power field.[95] However, in a retrospective study analyzing clinical records and skin biopsies from 108 patients with SJS, SJS/TEN overlap, and TEN, dermal infiltrate severity was not associated with day 1 SCORTEN or hospital death, but full-thickness epidermal necrosis was associated with mortality.[111]
Early Withdrawal and Half-Life of the Causative Drug: A 10-year observational study including 113 patients with TEN or SJS found that the earlier the causative drug was withdrawn, the better the prognosis was (odds ratio 0.69 for each day; 95 % confidence interval 0.53–0.89). Patients exposed to causative drugs with long half-lives had an increased risk of dying (odds ratio 4.9; 95 % confidence interval 1.3–18.9).[84]
4.2 Treatment
The treatment of SJS/TEN consists of a multidisciplinary approach, which includes the following important aspects (Table 3):
4.2.1 Identification and Withdrawal of the Culprit Drug. The medication history during the previous 2 months is documented and all suspected and non-essential medications are withdrawn.[99]
4.2.2 Transfer of the Patient to an Appropriate Unit. The patient is transferred to intensive care, a burn unit, or another specialty unit.[112, 113]
4.2.3 Supportive Care. Thermoregulation: Cutaneous thermoregulation is impaired by the compromised skin barrier; thus, increasing the room temperature to 28–32 _C is important, especially for patients with large amounts of epidermal detachment.[99, 114]
Airway Protection: The decision to intubate and ventilate is made on the basis of the following considerations: the extent of upper airway mucosal involvement, potential for airway obstruction, severity of respiratory distress, and anticipated analgesia and sedation requirements.
Fluid Replacement and Assessment of Fluid Balance: Fluid management differs from that of patients with burn injuries; fluid and electrolyte requirements are less than for burns of the same extent.[115]Fluid replacement with electrolyte solution (0.7 mL/kg/% affected area) and albumin solution (5 % human albumin, 1 mL/kg/% affected area) is advised.[99] The goal is to maintain a urinary output of 0.5–1 mL/kg/h.[115]
Nutritional Support: Enteral nutritional hypercaloric and high-protein diet support is advised to prevent protein loss and promote healing. Enteral nutrition through a nasogastric tube should be considered in patients unable to ingest food. Nasogastric feeding should be initiated cautiously because of possible gastrointestinal involvement and potential difficulties in placing a tube in the presence of oropharyngeal involvement.[116] Parenteral nutrition is not recommended, because it is commonly poor tolerated and is associated with an increased risk of sepsis.[114] Patients with SJS/TEN require fewer calories per day than patients with burns. A recommended equation for the estimation of energy requirements in pediatric SJS/TEN patients is (24.6 × weight in kg) + (% wound × 4.1) + 940.[117] Other guidelines advise the following protocol for nutrition through a gastric tube: 1500 calories in 1500 mL over the first 24 h, increasing by 500 calories daily to 3500–4000 calories daily.[99]
Pain Management: Management of pain is a major point, often underestimated, and necessitates assessment and medical treatment accordingly. Use of morphine should be considered, with appropriate respiratory monitoring. If available, patient-controlled analgesia is advised.[114] Placement of the patient on an alternating pressure air mattress may also reduce the pain.[99]
Venous Thromboembolism Prophylaxis: Standard prophylactic anticoagulation therapy is advised for patients who are bedridden.[114]
Monitoring for Infection: Antibiotic prophylaxis is not recommended. However, monitoring for infection is mandatory, and if clinical suspicion arises, bacterial cultures should be obtained from the skin, urine, and blood, and empirical treatment with antibiotics given until culture results are available.[99] Some guidelines advise performing bacterial and fungal cultures from the skin, urine, and blood on a regular basis (2–3 times per week).[112–114]
Psychological Support: Professional psychological support for the patient and family members is important.[99]
4.2.4 Medical Treatment. Systemic Immunomodulatory Treatment in Adults: The optimal therapeutic regimen has yet to be established, but, according to recent publications, the following conclusions can be drawn.
-
Intravenous immunoglobulin (IVIg): Use of IVIg does not yield survival benefits in adults with SJS/TEN.[118–120]
-
Cyclosporine: In an open-label, phase II trial to determine the safety and possible benefit of cyclosporine, cyclosporine was found to decrease the death rate and the progression of detachment in adults (dosage: 3 mg/kg/day for 10 days and tapered over a month).[121] Other publications have also reported beneficial use of cyclosporine in SJS/TEN.[122, 123]
-
Systemic Corticosteroids: These were associated with clinical benefit according to the EuroSCAR (European Study of Severe Cutaneous Adverse Reactions) study[124] and were reported to be the most common treatment for SJS/TEN in a recent survey of 50 drug hypersensitivity experts from 20 countries.[125] One of the suggested protocols is intravenous dexamethasone 1.5 mg/kg pulse therapy (given for 30–60 min) for three consecutive days.[126]
-
TNF Inhibitors: Treatment with anti-TNF biologic agents seems to be very promising for the management of SJS/TEN.[127, 128] In a recent study in ten consecutive patients with TEN, 50 mg of etanercept was administered in a single subcutaneous injection. All patients responded promptly to treatment, reaching complete re-epithelialization without complications or side effects.[129] In addition, the preliminary results of a prospective, randomized, open-label trial currently underway in Taiwan,[125] comparing etanercept with systemic corticosteroids in patients with SJS/TEN, demonstrated that the average time to reach maximal skin detachment and complete skin healing was shorter in the etanercept group. Also, in vitro investigations demonstrated that etanercept, corticosteroids, and thalidomide significantly decreased granulysin expression of blister cells. Etanercept did not, however, increase the cytotoxic effect on keratinocytes found with thalidomide.[125]
Systemic Immunomodulatory Treatment in Children: The bulk of the literature on the management of SJS/TEN includes adults, and these findings may not be simply extrapolated to children. On the basis of the scant quality literature in children, both IVIg and systemic corticosteroids seem to improve the outcome of children with SJS/TEN.[130–132]
Skin Treatment: There are no clinical guidelines for the skin care of patients with SJS/TEN. Debridement of the necrotic epidermis was recommended in past publications.[112, 133] Recent publications advise avoiding debridement (which may cause hypertrophic scars) and recommend considering the detached epidermis as a natural biological dressing that favors re-epithelialization.[64, 114, 125] Various topical treatments that have been reported include bioactive skin substitutes, semi-synthetic and synthetic dressings, and topical antimicrobials.[112, 133] A recent report on the management of SJS/TEN in an experienced French referral center described the following treatment: wound care once daily with minimal manipulation to prevent skin detachment, including a bath containing a solution of chlorhexidine 1/5000 (morphine is given prior to the bath and/or an equimolar mix of oxygen and nitrogen monoxide during the bath); if bathing is not possible, the chlorhexidine solution is sprayed 2–3 times daily on the skin; blister fluid is aspirated while maintaining the blister roof; petrolatum is systematically applied over all detached skin areas; topical sulfa-containing medications are avoided; and hydrocellular or absorbent nonadhesive dressings are applied at least once daily to cover pressure points.[114] Another recently published protocol[134] based the wound management algorithm on the stage of denudation and skin loss, categorizing four stages of skin with specialized treatments accordingly.
Mucous Membrane Treatment: Specialized care is essential to prevent lifelong complications.[64]
-
Ocular management: Although there is no standardized care for ocular management, the following supportive local treatment is advised: tear replacement solutions; removal of peudomembranes; lysis of symblepharon; debridement of loosened epithelium; topical antibiotics to prevent secondary infection; topical corticosteroids to prevent scar formation; and cycloplegic drops to relieve pain, photophobia, and ciliary spasm.[135] Amniotic membrane transplantation has been found to be effective in the acute and chronic stages of SJS/TEN.[136, 137] A ''Triple-TEN'' protocol for severe ocular cases was recently reported,[138] comprising the following:
-
Subconjunctival triamcinolone (Kenalog™ 20 mg) is administered into each of the fornices to curb the local inflammatory response without compromising systemic immunity.
-
Amniotic membrane tissue mounted on a polycarbonate skirt (ProKera®) is placed over the corneal and limbal regions to facilitate reepithelialization of the ocular surface.
-
A steeply curved acrylic scleral shell spacer (Technovent, SC21) is inserted to vault each lid away from the globe and provide a barrier to symblephara formation. This treatment offers an effective therapeutic option without the need for microsurgical equipment, a microscope, or sutures in the critical care setting.
-
-
Oropharyngeal Management: The mouth should be rinsed several times daily with an antiseptic or antifungal solution, and the lips should be lubricated with an ointment such as dexpanthenol.[99]
-
Genital Management: Vulvovaginal sequelae of SJS/TEN are well documented in the literature, though little consensus exists about effective preventive strategies. The goals of therapy should be to protect vaginal function by decreasing adhesion formation and agglutination, as well as limiting vulvovaginal adenosis with potentially neoplastic changes in affected tissue.[72, 139–141]Some treatment protocols suggest wet dressings or sitz baths and lubrication with emollient to avoid adhesions and strictures of genital erosions in females.[99, 114] In a recent expert opinion publication,[141] the authors advised using the following protocol:
-
Early initiation of intravaginal steroids: Betamethasone valerate 0.1 % cream applied every 12 h to the vulva externally, and betamethasone valerate 0.1 % ointment applied every 12 h to the internal vaginal mucosa via a Milex® dilator (Milex Products Inc., Chicago, IL, USA); regular application of antifungal creams as well. Treatment was advised to be given in alternating courses to prevent tachyphylaxis (for example, 3 days on, 4 days off), and treatment with topical steroids should be continued until resolution of the acute phase of the illness.
-
Vaginal molds: In addition to topical steroids, a soft vaginal mold should be placed prophylactically as early as possible during the acute phase of the illness and used regularly until complete healing of ulcerative lesions has occurred. The mold can be coated with topical steroids, and patients are advised to wear it for most of the day, removing it only for cleansing, medication application, and toileting. The author advised using Milex_ vaginal dilators; they are made of latex-free, hypoallergenic silicone and come in various lengths and widths. They are available to purchase from online distributors (e.g. CooperSurgical, Trumbull, CT, USA). Special consideration is to be given to virginal patients, in whom use of vaginal molds would be inadvisable. Instead, the steroid formulation can be applied via a standard vaginal applicator. Also, use of a dilator is not advised in the pediatric population, to prevent physical discomfort and emotional trauma.
-
Menstrual suppression: To decrease the risk of vaginal adenosis, menstrual suppression in the acute phase and healing process is advised. Detailed information on treatment of confirmed adenosis and the importance of close colposcopic surveillance, given the association of this process with subsequent malignancy, is described in this report.[141]
-
Special Considerations in Pregnant Women: Many cases of SJS/TEN occurring during pregnancy have been reported, with high survival rates of both the mothers and the newborns.[142–144]
The following major aspects are of special concern when treating a pregnant woman with SJS/TEN:
-
Fetal status: Fetal stress due to maternal disease may lead to premature labor.
-
Delivery methods: Although vaginal deliveries have been reported,[145] Caesarean section has been used in many cases[142, 144] because of genital mucous membrane involvement of TEN. Risk management for labor includes frequent obstetric examinations to assess possible vaginal strictures, which may impair spontaneous delivery. If a spontaneous delivery is planned, preparations for a Caesarean section should be made, and anesthetic and neonatology support should be available.[142]
-
Involvement of the disease within the fetus/newborn: SJS/TEN may develop during pregnancy in the mother alone or simultaneously in the mother and fetus; it can be lethal for the fetus.[142] In cases reported in the literature, most babies presented as healthy.[142, 144] However, a few cases of TEN in newborns, including one case of simultaneous TEN in a mother and her stillborn fetus, have been reported.[143]
-
HIV: Pregnancy increases the chances of developing SJS 14-fold in HIV-infected pregnant women treated with nevirapine.[146] A recently published retrospective study of patients admitted to a university hospital with SJS/TEN over a 3-year period in Cape Town, South Africa,[147] found that nevirapine-based antiretroviral therapy—a regimen for women in the first trimester of pregnancy and those planning a pregnancy to prevent mother-to-child transmission of HIV infection—placed pregnant women at a disproportionally higher risk of developing SJS/TEN.
Avoiding High-Risk Drugs and Monitoring for Secondary Cutaneous Adverse Drug Reactions: Avoiding high-risk drugs (Table 4) is important, as SJS/TEN predisposes the patient to developing additional cutaneous adverse drug reactions.
Monitoring and Treating Acute Complications: The most common cause of death in SJS/TEN in the acute stage is septicemia. Nonetheless, other possible complications induced by changes in the hemodynamic status and systemic involvement of SJS/TEN are to be monitored and treated.
4.2.5 Communication with the Patient, Family, Health Care Providers and Regulatory Agencies.Patient and Family: Good communication strategies will aid in the interactions with the patient and family following SJS/TEN and will decrease the likelihood of lawsuits. Physicians are advised to follow these steps:
-
Express empathy and say ''sorry'' according to the ''apology laws'' in an honest and respectful fashion, and in a way that protects the physician from having an apology used against him in case of legal action. See http://www.sorryworks.net/.
-
Provide disclosure in a ''disclosure meeting'' planned according to the acronym CONES (Context: arrange the setting for a quiet, uninterrupted meeting, and decide on the participants; Opening shot: the first sentence in the meeting explains the aim of the conversation; Narrative: lay out the facts, and avoid using the words ''error'' and ''mistake'', since the SJS/TEN is a result of multiple factors, particularly when the facts are not completely known; Emotions: provide an empathic environment; Summary).
-
Provide the patient with clear information about SJS/TEN and its cause, the name of the offending drug, potential cross-reacting drugs, and drugs that can be safely taken as an alternative to the offending drug. In addition, provide the results of the HLA genetic screening, if conducted, and advise the patient to wear a MedicAlert® bracelet.
-
Family counseling is part of the management plan because of genetic predisposition. The authors' protocol is to advise all first-degree family members to avoid use of the drug that caused the SJS/TEN in their family member (unless past treatment was safe),[3] and to provide information on HLA genetic screening for the relevant drug if available.
Health Care Providers: Information on the adverse event must be provided to the family physician and entered into the patient's records.
Regulatory Agencies: Report the cutaneous adverse drug reaction to the manufacturer and regulatory agencies.[148] International reporting systems include MedWatch, the FDA Safety Information and Adverse Event Reporting Program (http://www.fda.gov/safety/MedWatch/default.htm), and the WHO Uppsala Monitoring Centre (UMC; http://www.who-umc.org).
4.2.6 Prior to Discharge. Information on the Culprit Drug: This should be provided to the patient and their primary physicians. Appropriate labeling should be applied to the medical record, according to the most likely causative drugs. Care should be taken to verify that the appropriate coding has been applied (e.g. International Classification of Diseases, Tenth Revision [ICD-10] for future reference and studies.
Medical Recommendations: Advise on medical treatment, if still necessary, and provide clear recommendations on sun protection to prevent post-inflammatory hyperpigmentation.
Explanation of the Possible Long-Term Medical Complications and Information on Medical Follow-Up: Longterm physical complications of patients surviving SJS/TEN are common (see elaboration in Sect. 3). Hence, it is highly important to explain to the patient the prognosis and the need for follow-up.
Offering Emotional Support: We recommend using the General Health Questionnaire-12 (GHQ-12)[149]—the most commonly used screening instrument for psychiatric disorders in various settings—as a quick and valid screening instrument for psychological distress. Patients should be asked to complete the scale at discharge, and scores of 2 or greater should trigger a referral to a psychiatrist or psychologist.[94]
Referral to a Support Group: Several support groups have been established in different countries, such as the Stevens–Johnson Syndrome Foundation in the USA, CAST (Canadians Against Stevens Johnson Syndrome & Toxic Epidermal Necrolysis) in Canada, and Amalyste in France. More information on support groups in different countries can be found athttp://www.sjsupport.org/sjsupport_group_facilitators.shtml.Continue Reading
5 Conclusion
SJS/TEN is one of the few dermatological diseases that constitute a true medical emergency.
In the last decade, important progress has been achieved in our understanding of the pathogenesis, clinical presentation, and treatment of SJS/TEN. Furthermore, laboratory-based discovery of the HLA alleles associated with SJS/TEN induced by drugs has been translated into a clinical guideline-based test, which serves as a practical clinical tool for genetic screening to prevent SJS/TEN.
However, there is still no international consensus on the management of patients with SJS/TEN, and genetic screening is not well implemented in most countries.
This review has summarized up-to-date insights on SJS/TEN, including new discoveries on the genetic associations of SJS/TEN, and described a protocol for assessment and treatment. We hope that this summary will increase the awareness among physicians regarding the importance of genetic screening for decreasing the incidence of SJS/TEN and that our suggested management protocol will serve as a practical clinical tool for physicians.
Further research is needed to generate adequate in vitro and in vivo models for SJS/TEN, to develop novel treatments, to study the involvement of unknown pathogens, and to further investigate the mechanism of keratinocyte death.




